Polymer Cross-Linking
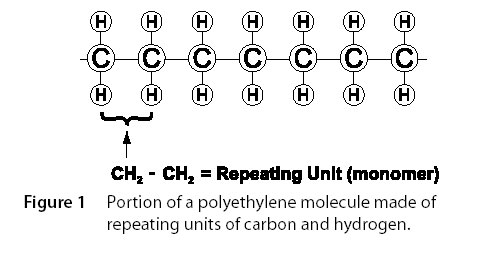
All plastic materials consist of complex, very large molecules. In the case of those compounds used as insulation and jacketing materials, they are called polymers. A polymer is a large molecule built up by repetition of many, smaller chemical units. Polyethylene consists of a long chain of units, each made of one carbon atom with two associated hydrogen atoms. Figure I illustrates a portion of a polyethylene molecule. An actual molecule is approximately 5000 units in length.
Polyethylene consists of many random molecular chains with no particular orientation and no chemical bonds existing between chains. When heat is applied to such a material, the chains are free to slip and flow under relatively small outside force. Such a material is called a thermoplastic. If we are able to introduce cross-linking bonds between adjacent molecular chains, this adds form stability at higher temperatures. There will still be some loss of strength at elevated temperatures, but the crosslinked molecular chains are much more resistant to flow when stress is applied.

Contact one of our experts today to learn more about our cables and wire, or to discus your cable and wire needs at:
Crosslinking can be accomplished chemically or by irradiation. Chemical crosslinking with rubber material is called Vulcanization. It is accomplished by a heat induced reaction between the polymers and a crosslinking agent. For wire and cable insulations, chemical crosslinking is performed by passing the wire through a long pressurized steam tube, called a continuous vulcanizing (C.V.) machine. Other methods of crosslinking include: Moisture cure, salt cure, and hot air vulcanization (HAV).
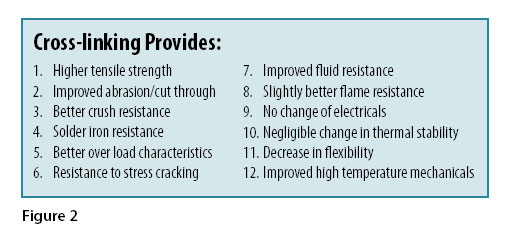
Not all materials can be crosslinked, but for those that are able, the results are important. Figure 2 summarizes the effects that polymer crosslinking provides.
In general, mechanical characteristics are improved, especially at higher temperatures. It results in improved resistance to stress cracking and better fluid resistance. There is generally little or no change in flame resistance, electrical characteristics, or thermal stability. One of the most misunderstood results of crosslinking is its effect on long term thermal stability. Uncrosslinked polyethylene is usually rated at 75°C, not due to heat aging, but due to the fact that the material becomes soft and will flow at higher temperatures. Properly compounded and crosslinked polyethylene may have a temperature rating as high as 125°C. The material no longer flows at elevated temperatures and therefore can be effectively used at a higher temperature.
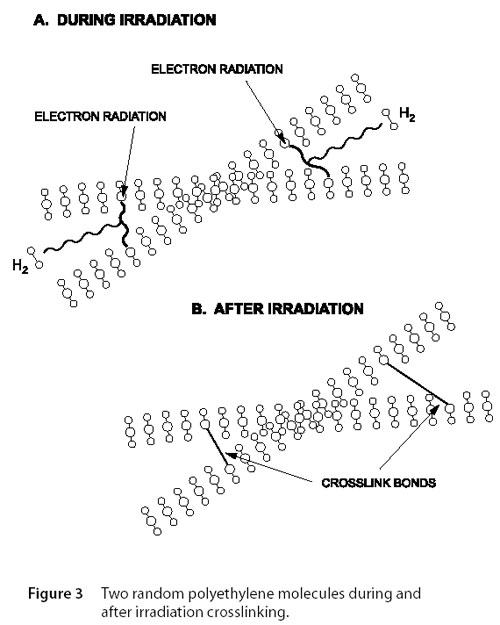
Polymers may also be crosslinked by means of electron irradiation. During the irradiation process, high energy electrons bombard the insulation system. Figure 3 illustrates two random polyethylene molecules being subject to irradiation. The energy of irradiation ejects a hydrogen atom which then removes a neighboring hydrogen atom, forming molecular hydrogen gas (H2). The vacant sites on the adjacent polymer chain then combine to create a crosslink bond.
For most plastic materials, equivalent properties may be obtained by the use of either C.V. or irradiation crosslinking, but irradiation may have the following advantages:
1. Irradiation has no lower limit on physical size, smaller conductor sizes, and thin insulation walls may be provided.
2. Irradiation does not use high temperature or pressure. Separator tapes are not required to prevent thin wall insulations from being forced into the conductor strand surface.
3. Irradiation offers the insulation compounder design freedom. Compound additives may be chosen without regard to their reaction to high temperatures and to moisture.